by Simon Elliott, Director of Atomic Level Process Simulation, Schrödinger
Challenges in predicting volatility
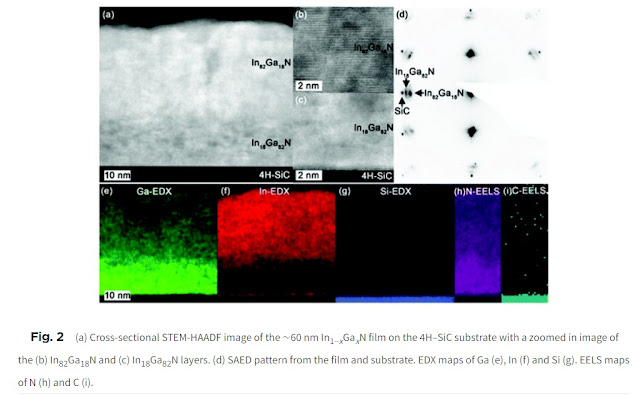
A crucial process in manufacturing CPUs and other high-tech devices is the deposition of solid material from reactive vapors. Different precursor vapors are used for chemical vapor deposition, vapor phase epitaxy, atomic layer deposition – and indeed the reverse process of atomic layer etching – with the precursor chemistry carefully designed for each case so as to control material quality at the nanoscale. But what all these techniques have in common is that the precursor chemicals must evaporate or sublime at a low enough temperature. Too much heating when vaporizing a precursor can make it decompose, causing it to be undeliverable to the growing surface.
With volatility playing such a central role in this technology (and in other fields like distillation, refrigeration, inkjet printing, food, and perfumes), it is surprising that we understand so little about it. Volatility is the product of a remarkably fine balance of interatomic forces, dictating the extent to which molecules condense together as a solid or liquid, or bounce apart into a vapor and deliver a certain vapor pressure at any given temperature. These interatomic forces can be computed very precisely with quantum mechanics for one molecule or a group of molecules, but not at the scale of a liquid or solid. Even with today’s computing power, routinely and accurately predicting precursor volatility ‘from first principles’ remains unfortunately out of reach.
Machine
learning approaches
Could an alternative more empirical approach prove useful? Does enough experimental data exist to find the relation between volatility and chemical structure? The vaporization of some organic molecules, such as alcoholic fractions or natural fragrances, has been of interest for centuries and high-quality vapor pressure data are available in the literature. Over the last decade, these data have been analyzed with advanced fitting algorithms that come under the umbrella of ‘machine learning’. Schrödinger has leveraged the latest machine learning techniques to develop a highly accurate model that predicts the volatility of organic molecules up to C20.
However, when building machine learning models to predict volatility of precursor molecules, which are typically organometallic complexes, the situation is not so straightforward. New precursor molecules are constantly being proposed and evaluated. Commercial sensitivity sometimes means that data are partially withheld or plagued by experimental configuration differences from laboratory to laboratory. Additionally, for the common aim of material processing, complete pressure-temperature curves are rarely measured, as it is more pragmatic to focus on the temperature for vapor to transport successfully to the reactor. As a result, datasets for building predictive models are sparse and incomplete.
Prediction
of volatility for inorganic and organometallic complexes
Schrödinger scientists embarked on the challenge of building machine learning models to predict the volatility of precursor molecules. Using in-house expertise in machine learning and advanced informatics, Schrödinger scientists collated and digitized information about organometallic precursors from disparate literature sources and applied a variety of machine learning algorithms (such as Random Forest and Neural Networks) in conjunction with different chemoinformatic descriptors and fingerprints. The result is the first capability of its kind for accurately and efficiently predicting the volatility for inorganic and organometallic complexes from their chemical structures. For complexes of the fifty most common metals and semimetals, the model predicts the evaporation or sublimation temperature at a given vapor pressure with an average accuracy of ±9°C (which is about 3% of the absolute temperature). As a trained model, the turnaround time is fast with the ability to compute hundreds of complexes per second.

New
avenues for precursor development
This predictive model opens a new path for designing novel precursors with improved performance, not only improving their deposition or etch chemistry, but also optimizing the temperature at which they evaporate or sublime and can be delivered as a vapor. This advance will allow a much wider range of structural modifications to be screened computationally than before and will produce candidate precursors for experimental synthesis and testing that are both less risky and more innovative. This volatility model, together with Schrödinger’s quantum mechanics-based workflows for computation of reactivity and decomposition, gives scientists a complete design kit for vapor-phase deposition or etch, delivering a faster pace of research into materials and processes for new technologies.
* The banner image is from Tyndall National Institute.
About the author
Dr. Simon Elliott is Director of Atomic Level Process Simulation at Schrödinger. From 2001-2018 he led a research group at Tyndall National Institute, Ireland. Prior to that, he studied chemistry at Trinity College Dublin and Karlsruhe Institute of Technology. He qualified as a Project Management Professional and is a Fellow of the Royal Society of Chemistry. He was co-chair of the 16th International Conference on Atomic Layer Deposition and chair of a 175-member COST network on the same topic.
About Schrödinger
Schrödinger is an industry-leading computational solutions provider for both life science and materials science, with a mission to improve human health and quality of life by transforming the way therapeutics and materials are discovered.
With the goal to accelerate the discovery and optimization of novel materials by a digital chemistry platform governed by physics-based modeling, amplified by machine learning, and optimized through team-based intelligence, Schrödinger’s Materials Science platform offers unprecedented insights into the mechanisms and properties of materials and chemical systems in a wide range of technological applications: Organic Electronics, Polymeric Materials, Consumer Packaged Goods, Catalysis & Reactive Systems, Semiconductors, Energy Capture & Storage, Complex Formulations, Metals, Alloys & Ceramics.
Learn more
Request more
information



%20(1).png)