Strem Chemicals
is a well-established (since 1964) supplier of ALD and CVD precursors for both
R&D and industrial applications. Many of their compounds are also available
in electronic grade suitable for semiconductor applications. The full range of
their ALD and CVD precursors can be found in their famous catalog available as
a hard copy or on line [LINK]. Amongst the
wide range of precursors, the platinum precursors and especially the (trimethyl)methyl-cyclopentadienylplatinum(IV)
- MeCpPtMe3 has proven popular for a wide range of ALD and CVD applications.
Platinum and
platinum-rich alloys are naturally occurring and have been known for a long
time since it is often found as native platinum. It occurs naturally in the
sands of rivers in South America and it was first used by pre-Columbian natives
to produce artifacts. Later in 16th century the Spaniards named the metal
"platina," or little silver, when they first encountered it in
Colombia. They regarded platinum as an
unwanted impurity in the silver they were mining and it was not until 1748 that
platinum was properly reported by Antonio de Ulloa y de la Torre-Giral, a
Spanish general of the navy, explorer, scientist, author, astronomer and
colonial administrator.
Since the
platinum has become known and used because of the outstanding catalytic properties,
which it has in common with the other of the six platinum group metals (PGM) –
iridium, osmium, palladium, platinum, rhodium, and ruthenium. In
addition, platinum's wear and tarnish resistance characteristics are well
suited for making fine jewelry. Other distinctive properties
include:
- high resistance to chemical attack
- excellent high-temperature characteristics
- stable electrical properties.
Because of all
these extraordinary properties the PGMs have been exploited for a wide range of
industrial applications. Platinum, platinum alloys, and iridium are
used as crucible materials for the growth of single crystals, especially
oxides. The chemical industry uses a significant amount of either
platinum or a platinum-rhodium alloy catalyst to catalyze the partial oxidation
of ammonia to yield nitric oxide, which is the raw material for fertilizers,
explosives, and nitric acid.
In recent years,
a number of PGMs have become important as catalysts in synthetic organic
chemistry. Platinum supported catalysts are used in the refining of crude
oil, reforming, and other processes used in the production of high-octane
gasoline and aromatic compounds for the petrochemical
industry. Since 1979, the automotive industry has emerged as the number
one consumer of PGMs. Palladium, platinum, and rhodium have been
used as oxidation catalyst in catalytic converters to treat automobile exhaust
emissions. A wide range of PGM alloy compositions are used in
low-voltage and low-energy contacts, thick- and thin-film circuits,
thermocouples and furnace components, and electrodes.
It was not until
the early 2000 that the platinum and the other PGMs became available as a ALD
processes and here below is a summary of the most important fundamental discoveries
of platinum ALD.
Thermal ALD of high quality platinum films
It all started
with thermal ALD of platinum and ruthenium in Helsinki Finland at the famous
Laboratory for Inorganic Chemistry headed by Prof. Markku Leskelä and Prof.
Mikko Ritala. Here it was found that high quality platinum films can be grown
by thermal ALD from MeCpPtMe3. According to the first publications
by Titta Aaltonen (summarized in her PhD Thesis University of Helsinki: LINK) the
films had strong (111) orientation even down to the lowest growth temperatures.
Except for discovering the secrets of thermal ALD of noble metals (Ru, Ir Pt,
Pd) Titta Aaltonen made groundbreaking studies of their ALD growth mechanism with O2 as the
co-reactant. At first it may seem strange that O2, or in her case also
laboratory air or pressured air, could be used to grow high quality noble metal
films. Titta Aaltonen found that adsorbed oxygen atoms react with the ligands
of the noble metal precursor during the metal precursor pulse. Unreacted ligand
species that remained on the surface after the metal precursor pulse react with
oxygen during the following oxygen pulse. The main reaction by-products
detected during the both reaction steps were water and carbon dioxide. For
detailed studies of the ruthenium process using RuCp2 it has been
concluded that active oxygen that dissolves in the upper most monolayers of the
growing noble metal film may be behind the nucleation and growth mechanism of
the next “ALD monolayer”.
The growth rates
of the platinum films grown at 300 °C from MeCpPtMe3 was reported at
about 0.5 Å/cycle both when air and pure oxygen were used as oxygen sources and
a 50-nm film grown at 300 °C had a resistivity of 13 μΩcm, which is close to
bulk value for platinum. It was also found that the difference between air and
O2 co-reactant was in how the films adhered to the substrate. The films grown
with air as the oxygen source did not pass the famous scotch tape test, while
the films grown with pure oxygen passed the tape test.
Besides having
such a beautiful ALD mechanism with such a simple co-reactant as air or O2,
one additional very big advantage with the MeCpPtMe3 precursor is
that can be vaporized at room temperature, just slightly below its melting
point of 30 °C since the vapor pressure of MeCpPtMe3 at room
temperature is high enough for delivery into an ALD process chamber. If you
need a bit more precursor flow for larger batch type reactors or applications
with relying on high surface area you can melt the precursor in a standard
stainless steel ampule or bubbler with carrier gas dip tube to enhance the flow
further.
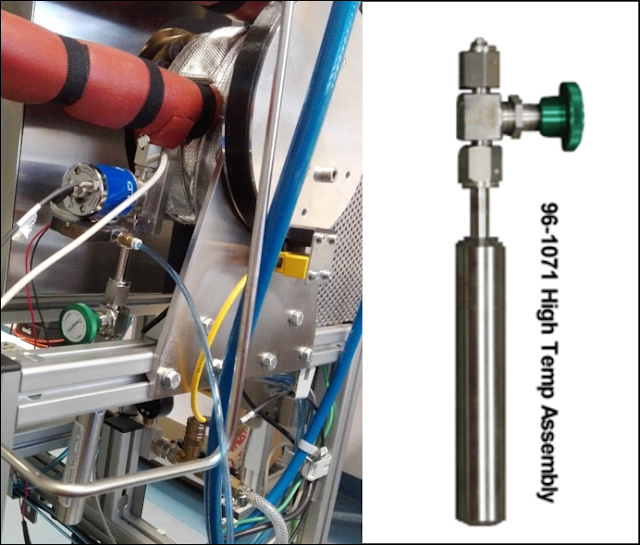
A hook up of MeCpPtMe3
precursor supplied in a Strem
Swagelock ALD/CVD cylinder via a standard Swagelock ALD-valve as close as
possible to a thermal horizontal low pressure ALD/CVD reactor (at Fraunhofer
IKTS, Dresden, Germany, LINK) to
save valuable platinum precursor (LINK) In
order to enhance the precursor flow the installation can be wrapped with heater
tape and heated to 30-50 °C.
Plasma ALD of platinum films
Some years later,
Harm Knoops (now TU Eindhoven/Oxford Instruments) and co-workers published
extensive results in a benchmarking study in 2009 [LINK]
using MeCpPtMe3 precursor in a plasma ALD reactor with a remote ICP
O2 Plasma. Here they proved that by the plasma enhanced ALD process
(PEALD), the growth temperature could be reduced considerably to as low as 100
°C for both platinum metal and platinum oxide film growth and it was possible
to switch between the two growth modes by adding a H2 step to grow
metallic films. More recently, the same group reported platinum ALD at room
temperature on polymer, textile, and paper substrates [LINK]. By
tuning the dosing of MeCpPtMe3, O2 plasma exposure, and H2
gas or H2 plasma exposure high-quality platinum films with a
resistivity of 18–24 μΩ cm were obtained.
Growth of platinum nanoparticles by ALD
Most recently
Prof. Ruud van Ommen (TU Delft) published their detailed study [LINK] on
how to control and grow platinum nanoparticles by ALD, again using the
MeCpPtMe3 precursor.
They showed that
the nanoparticle aggregation takes place during the oxygen half-reaction and
that the mobility of the nanoparticles exhibits a size- and
temperature-dependent scaling and that ALD-like precision over the nanoparticle
size requires low deposition temperatures (< 100 °C).
Industrial applications for platinum ALD
Since early 2000
platinum ALD has been considered in parallel to ruthenium and evaluated
multiple times by academia and industry for the use in a number of
microelectronic applications including:
- Electrodes for DRAM high-k capacitors
- Transistor Source/Drain contacts with nickel Ni(Pt)Si
- DRAM buried Word Lines and Bit Lines
- Local interconnects as Cu seed layer or complete fill replacing tungsten
The semiconductor
industry is very sensitive for raw material pricing and therefore introduction
of platinum so far has mainly been using PVD in the case of Ni(Pt)Si source
drain contact and for the other applications mentioned above there has been no
reports of high volume manufacturing. Meanwhile, ruthenium on the other hand
had have some success for hard disk reader heads and is now considered for
local interconnects for technologies at 5 nm or below.
One of the
biggest industrial applications for the MeCpPtMe3 precursor today is for E-beam
direct write repair of photo lithographic masks for both Immersion and EUV
lithography and making direct chip level contacts for electrical
characterization in FIB-SEM.
Current research and development on using platinum ALD or CVD as deposition method focuses on:
- Nanobatteries using platinum contacts and electrodes
- Supercapacitors using platinum electrodes
- Nanoparticle catalysis
- Core shell nanoparticles (nanoparticles covered by an ultra-thin platinum layer)
- As contacts to III/V nanowire and 2D materials devices
- Electrodes and contacts in printed flexible electronics
- 3D Nanoprinting via laser-assisted electron beam induced deposition
The main issue to
overcome for any successful industrial scale up of platinum is to minimize the
use of bulk platinum and use ultra-thin layers and if bulk material is need use
either substrates with a very large surface or coated low cost particles.
Eventually, for all applications, platinum being a noble metal all of the by-products
of precursor or coated parts has to be recaptured and recycled.
In the case of
automotive catalyst support such PGM recycling plants are operational since
long time (e.g. operated by BASF and Umicore). For the ruthenium introduction
in the semiconductor device manufacturing, several companies have reported
development of recapture and recycling methods (e.g. Praxair, Tokyo Electron
and Tanaka) and we can assume that these can also be adapted for platinum
precursor recapture and recycling. Finally, to put things in perspective, the
USGS reported that about 110,000 kilograms of platinum, palladium, and rhodium
was recovered globally from new and old scrap in 2017 and they estimate the
world resources of PGMs to a total more than 100 million kilograms. The largest
reserves are in the Bushveld Complex in South Africa.
References
ALD of platinum from MeCpPtMe3 and Air and the ALD
nobel metal / oxygen reaction mechanism: T. Aaltonen, A.
Rahtu, M. Ritala, and M. Leskelä, Reaction Mechanism Studies on Atomic Layer
Deposition of Ruthenium and Platinum, Electrochem. Solid-State Lett., 6 (2003)
C130–C133. [LINK]
ALD of platinum from MeCpPtMe3 and O2 : T.
Aaltonen, M. Ritala, Y.-L. Tung, Y. Chi, K. Arstila, K. Meinander, and M.
Leskelä, Atomic Layer Deposition of Noble Metals: Exploration of the Low Limit
of the Deposition Temperature, J. Mater. Res., 19 (2004) 3353–3358. [LINK]
PEALD and thermal ALD of platinum films from MeCpPtMe3
: H. C. M. Knoopsa,
A. J. M. Mackus, M. E. Dondersa, M. C. M. van de Sanden, P. H. L. Notten, and
W. M. M. Kessels.
PEALD of platinum at room temperature : A.
J. M. Mackus, D. Garcia-Alonso, H. C. M. Knoops, A. A. Bol, and W. M. M.
Kessels, Room-Temperature Atomic Layer Deposition of Platinum, Chem. Mater.,
2013, 25 (9), pp 1769–1774 [LINK]
Platinum nanoparticle ALD growth : F. Grillo, H.
Van Bui, J. A. Moulijn, M. T. Kreutzer, and J. R. van Ommen, Understanding and
Controlling the Aggregative Growth of Platinum Nanoparticles in Atomic Layer
Deposition: An Avenue to Size Selection, J. Phys. Chem. Lett., 2017, 8 (5), pp
975–983 [LINK]
Facts about PGMs : Platinum-Group
Metals Statistics and Information (Platinum, Palladium, Rhodium, Ruthenium,
Osmium, and Iridium), U.S. Department of the Interior, U.S. Geological Survey [LINK]
MeCpPtMe3 product information and ordering from
Strem Chemicals (Item #: 78-1350):
Product Description:
(Trimethyl)methylcyclopentadienylplatinum(IV), 99%
CAS #: 94442-22-5
Safety Data Sheet: [LINK]



%20(1).png)