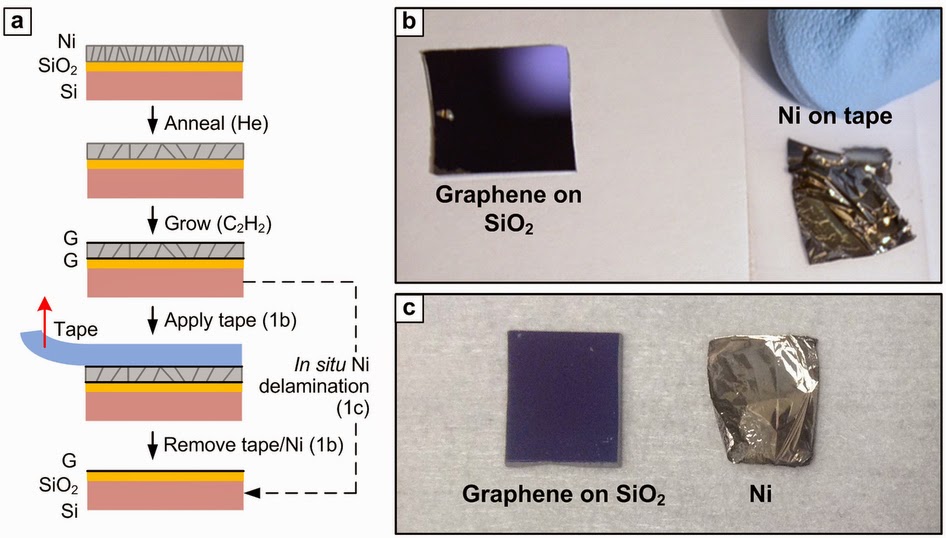
Alabama Graphite is pleased to announce that it has found naturally occurring flake graphene at its Coosa Property in Alabama, USA. The graphene was obtained using an innovative and cost effective process, by Dr. Nitin Chopra of The University of Alabama under our sponsored research partnership.
Graphite is made up of multiple layers of graphene stacked on top of each other. Graphene is a single layer of two dimensional (2-D) carbon atoms. Graphene is valued because it exhibits superior electrical, optical, mechanical and thermal properties. It is not only the strongest material known (200 times stronger than steel), but is also one of the most flexible.
“We believe that the discovery of naturally occurring single and multi-layer graphene, on the Coosa Property opens a completely new and unique business dimension for the Company,” stated Ron S. Roda, CEO of Alabama Graphite. “The biggest challenge today for commercial viability of graphene is cost. This presents a very exciting opportunity for our Company.”
“In my opinion, emerging technologies using graphene could greatly benefit from a cost effective processing methodology, with the potential for improved economics and increased production levels relative to any of the current methods used to create synthetic graphene,” commented Dr. Nitin Chopra. “The work done on the Company’s material has the potential to enhance the process of producing scalable, nano-manufactured graphene and graphene-based derivatives.”
Synthetic graphene is currently produced using a variety of expensive, tedious methods that do not lend themselves to large-scale production and are prone to produce defective graphene with uncontrolled flake size. Current synthetic methods for developing graphene include chemical vapor deposition (CVD), mechanical exfoliation, solution exfoliation, and chemo-mechanical methods. This implies higher costs including greater energy consumption, and extended manufacturing time.
The Company and Dr. Chopra continue to jointly develop methodologies to isolate graphene and graphene-based applications. Graphite flakes thinner than 100 nm are of significant interest because of their physical characteristics. Such thin graphite flakes ranging from one 2-D layer of carbon atoms (graphene) or multiple layers of 2-D carbon atoms stacked over each other (multi-layer graphene or graphite nanoplatelets) are of particular interest for developing advanced applications.
As shown in Figure 1 below, a moderately-sized (<5 μm, top right inset) single crystal flake of graphene, from the Coosa Property, is observed with a clearly visible carbon atom arrangement at high resolution (bottom right inset in Figure 1A). These flakes demonstrated very high quality Raman spectral features (G-band intensities, Figure 1B) with the ratio of disordered carbon signature to graphitic carbon signature of around ~0.15±0.05 (ID/IG). In addition, electron transparent flakes (bi-layer and multi-layer graphene) were observed in the analyzed samples.
“We believe that the discovery of naturally occurring single and multi-layer graphene, on the Coosa Property opens a completely new and unique business dimension for the Company,” stated Ron S. Roda, CEO of Alabama Graphite. “The biggest challenge today for commercial viability of graphene is cost. This presents a very exciting opportunity for our Company.”
“In my opinion, emerging technologies using graphene could greatly benefit from a cost effective processing methodology, with the potential for improved economics and increased production levels relative to any of the current methods used to create synthetic graphene,” commented Dr. Nitin Chopra. “The work done on the Company’s material has the potential to enhance the process of producing scalable, nano-manufactured graphene and graphene-based derivatives.”
Synthetic graphene is currently produced using a variety of expensive, tedious methods that do not lend themselves to large-scale production and are prone to produce defective graphene with uncontrolled flake size. Current synthetic methods for developing graphene include chemical vapor deposition (CVD), mechanical exfoliation, solution exfoliation, and chemo-mechanical methods. This implies higher costs including greater energy consumption, and extended manufacturing time.
The Company and Dr. Chopra continue to jointly develop methodologies to isolate graphene and graphene-based applications. Graphite flakes thinner than 100 nm are of significant interest because of their physical characteristics. Such thin graphite flakes ranging from one 2-D layer of carbon atoms (graphene) or multiple layers of 2-D carbon atoms stacked over each other (multi-layer graphene or graphite nanoplatelets) are of particular interest for developing advanced applications.
As shown in Figure 1 below, a moderately-sized (<5 μm, top right inset) single crystal flake of graphene, from the Coosa Property, is observed with a clearly visible carbon atom arrangement at high resolution (bottom right inset in Figure 1A). These flakes demonstrated very high quality Raman spectral features (G-band intensities, Figure 1B) with the ratio of disordered carbon signature to graphitic carbon signature of around ~0.15±0.05 (ID/IG). In addition, electron transparent flakes (bi-layer and multi-layer graphene) were observed in the analyzed samples.
Figure 1A) High resolution TEM image of single-layer graphene. Inset
(top right) shows low-resolution image of single-layer graphene. Inset
(lower right) shows atomic scale TEM image indicating arrangement of
carbon atoms (red hexagons) with bond length closely matching that of
C-C in graphene network.
Figure 1B) Raman spectra for various graphene flakes showing
significantly large G-band peak intensity as compared to D and 2D band.
This also corresponds to very low ID/IG ratio of ~0.15 ± 0.05
Rick Keevil, P. Geo., a Director of the Company and VP of Project Development, is a Qualified Person as defined by National Instrument 43-101, has approved the disclosure of the scientific or technical information concerning the Coosa Property contained in this press release.



%20(1).png)