Nanexa and Moderna have entered into a license and option agreement covering the development of up to five undisclosed drug compounds using Nanexa’s PharmaShell® drug delivery platform. The agreement includes an upfront payment of USD 3 million to Nanexa, with the potential for up to USD 500 million in development and commercial milestone payments, as well as tiered single-digit royalties on future product sales. Moderna receives an immediate license for the first selected compound and holds options to license up to four additional compounds following preclinical evaluation.
“We are excited to partner with Moderna, a pioneer and leader in the field of mRNA medicines, to explore the potential of our PharmaShell® platform and to support the development of improved products for Moderna,” said David Westberg, CEO of Nanexa. “This agreement underscores the versatility of PharmaShell and its potential to address key challenges in the delivery of advanced biologics.”
Background on ALD and PharmaShell
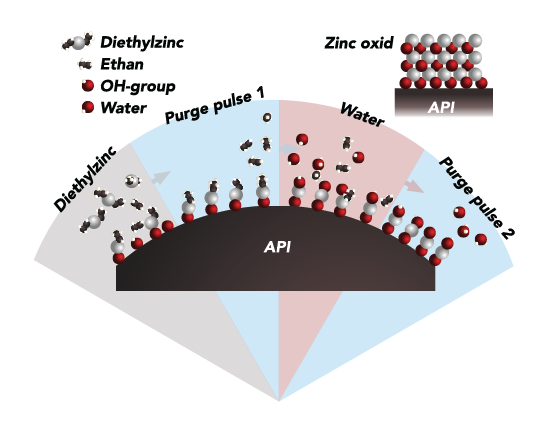
Atomic Layer Deposition (ALD) is a thin-film deposition technique originally developed for the semiconductor industry, where it is used to create extremely uniform, conformal, and precisely controlled coatings at the atomic scale. ALD is based on sequential, self-limiting surface reactions, which allow film thickness and composition to be controlled with angstrom-level precision. Because the process produces highly uniform coatings even on complex, high–surface-area structures, ALD has increasingly been adopted in life sciences and pharmaceutical applications where consistency, stability, and reproducibility are critical.
PharmaShell® is Nanexa’s proprietary drug delivery technology that applies ALD to pharmaceuticals by encapsulating active pharmaceutical ingredients with an ultrathin, inorganic coating. This coating acts as a controlled diffusion barrier, enabling precisely tuned and long-acting release profiles while also improving product stability and protection of sensitive molecules. By adjusting coating thickness and material properties at the atomic level, PharmaShell® can be tailored to specific drugs and therapeutic needs, making it particularly well suited for advanced biologics and long-acting injectable formulations.
PharmaShell® is administered as a suspended injectable formulation containing API particles coated with an ultrathin ALD-based shell. After injection, the coating gradually dissolves in vivo, enabling controlled and sustained release of the active pharmaceutical ingredient into systemic circulation. The coating materials break down into ions that are naturally eliminated via urine and feces, allowing long-acting drug delivery using thin-gauge needles and low injection volumes.
PharmaShell® is created by applying ultrathin inorganic oxide coatings to API particles using atomic layer deposition (ALD). ALD is a gentle, gas-phase process operating under dry conditions near room temperature, making it suitable for sensitive molecules such as peptides and monoclonal antibodies. The nanometer-scale coating provides precise control of drug release while maintaining a very high drug load, with no need for post-process purification.



%20(1).png)