BROOMFIELD, Colorado – Nov. 4, 2016 – Today, ALD NanoSolutions (ALD
Nano), the pioneer and market leader in Atomic Layer Deposition (ALD)
technology on particles, reported a banner year on multiple fronts. The
company partners with leading global materials companies to
commercialize ALD advanced materials that significantly improve the
performance, safety and other characteristics of end products in
industries like lighting, batteries, sensors, life sciences and
catalysts. 2016 highlights include new patents, deeper customer
engagements, expanded manufacturing space, and new reactors to increase
production capacity. The momentum illustrates how ALD Nano is harnessing
the immense near-term market opportunities for its proprietary ALD
technologies outside of ALD’s traditional deployment in the
semiconductor industry.
Leading with Differentiated Intellectual Property (IP)
Major 2016 milestones reinforced ALD Nano’s pioneering development
and leadership in ALD for control of surface properties at the atomic
level for unique functionality of particles and other materials. The
company obtained new patents, including some from the University of
Colorado Boulder (CU Boulder), its R&D partner since inception. This
brings ALD Nano’s total patent holdings to 28 issued and 14 pending.
The new IP heightens the market value and cost-effective use of its
“Particle ALD” and “Polymer ALD” to create advanced materials.
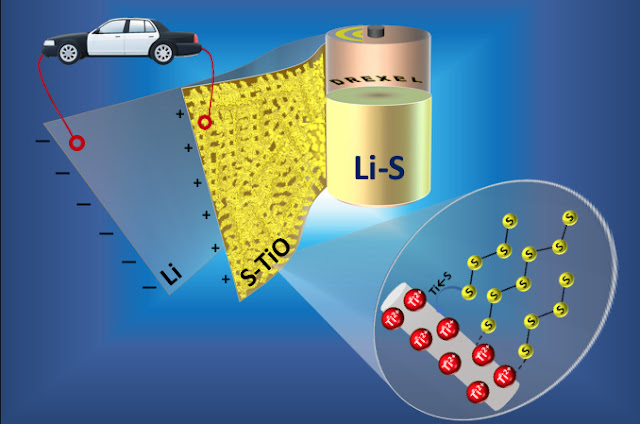
An important new patent1 covers an ALD method to deposit
inorganic films on organic polymer surfaces. For industries like OLED
displays and lithium-ion batteries, the innovation promises breakthrough
benefits that could displace other technologies. The Polymer ALD
technology could better protect battery electrode separators from
overheating and enable next-generation life-science tools, among other
applications.
Another new patent2 is for Particle ALD use with super capacitor electrodes, and an in-license3
from CU Boulder for additional applications of ALD for batteries.
Together, they strengthen the company’s position in the energy storage
market. A further patent4 covers the use of an ALD method to
apply a ceramic coating to implantable medical devices. This expands ALD
Nano’s position in the life sciences industry. The company also filed a
patent5 internationally for its revolutionary Particle ALD
continuous flow reactor system. This allows for large-scale,
cost-effective Particle ALD advanced materials production.
Enabling Innovation for Manufacturers of Lithium-Ion Batteries and LED Lighting
A standout 2016 highlight was the first commercial application of
Particle ALD for Cathode Active Materials (CAMs) used to produce
lithium-ion batteries. The breakthrough was achieved thanks to CU
Boulder’s extensive R&D and ALD Nano’s proprietary and robust IP
portfolio, coupled with the company’s strategic partnership with a
leading battery materials company. Particle ALD is the most effective
surface modification method available for CAMs. The ALD-enabled CAMs
will dramatically improve performance, extend cycle life and enhance the
safety of batteries for use in consumer electronics, electric vehicles
and grid storage.
Also in 2016, the company began commercial production of Particle ALD
phosphors for a Fortune Global 500 customer, following a multi-year
collaboration. The ALD advanced material significantly extends the
brightness lifetime for LED lights, while using a fraction of the
coating material required for other deposition methods.
Expanding Infrastructure to Address Growing Demand for ALD Solutions
With its accumulating IP, ALD Nano is expanding and deepening
engagements with customers. To support the momentum, the company doubled
manufacturing space at its headquarters in Colorado, and added new
reactors to increase production capacity. Headcount has also grown in
the last 12 months.
CEO Mike Masterson called 2016 a transformative year for ALD Nano:
“Our growth this year coincides with the consistently superior
performance of our ALD technology in many markets. This validates our
early vision and is now guiding our execution strategy to create ALD
advanced materials in partnership with leading sales channel partners
and customers. We’ll enter 2017 firmly positioned with differentiated
technology and expertise to help such companies achieve their technology
and cost-of-production goals. Our growth is a tribute to the steady
efforts of our team, and the extraordinary innovation contributed by
each individual.”
New ALD Nano Patents
1 US Patent 9,376,750
2 US Patent 9,406,449
3 US Patent 9,196,901
4 US Patent 9,279,120
5 US Application 62/175,964
About ALD
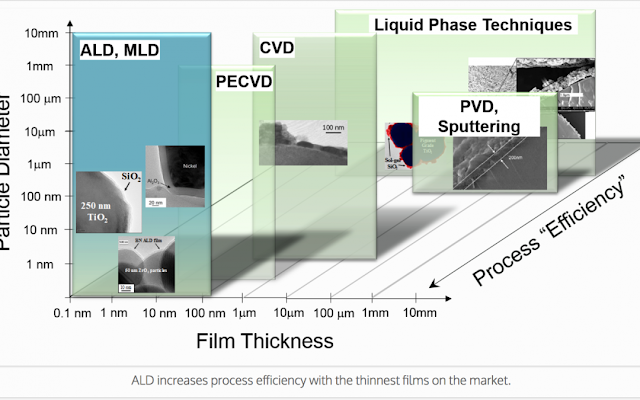
ALD is the sequential vapor phase material deposition method that forms
chemically bonded, high-purity, conformal, ultra-thin films of
controlled nanometer thickness. ALD generates less waste than other
deposition techniques such as chemical vapor deposition, giving
customers a sustainable and cost-of-ownership edge, while helping to
reduce overall costs. The atomic level precision of ALD on particles,
polymers and other substrates enables new or better applications of
materials resulting in ALD advanced material solutions. Devices such as
consumer electronics are getting smaller and more complex, requiring
novel materials to solve critical issues for marketplace adoption.
About ALD NanoSolutions
ALD NanoSolutions (ALD Nano) is creating cost-effective advanced
materials that are transforming industries such as lighting, energy
storage, consumer electronics, life sciences, fuel catalysts, water
purification, sensors, and more. We’re the leader in Atomic Layer
Deposition (ALD) technology on particles, with broad IP covering
polymers and MEMS, as well. We partner with world-leading companies that
leverage our material designs and reactor systems to innovate products
that benefit consumers globally. For more than a decade, we have
commercialized innovative ALD technologies developed internally and
through research conducted at the University of Colorado Boulder. We’re
headquartered in Broomfield, Colorado.



%20(1).png)