Copper replaced Aluminum for
interconnects in the semiconductor industry due to its low resistivity,
high resistance to electromigration, low temperature coefficient of
resistance, and good thermal stability [1].
Due
to the lack of volatile copper compounds, copper could not be patterned
by the techniques of photoresist masking and plasma etching that had
been used for aluminum. The inability to plasma etch copper meant that
the whole metal patterning process had to be redesigned and the result
was a process referred to as an additive patterning, also known as a
"Damascene" or "dual-Damascene" process by analogy to a traditional
technique of metal inlaying. [2]
However,
the exposed Cu interconnects during via-opening and post CMP process
are vulnerable to oxidation with water rinse and exposure to air,
resulting in reliability degradation [3]. Therefore, additional process
for reduction of copper oxide should be required. The cleaning of copper
can be achieved by either physical Ar sputtering or chemical reduction
process [4]. Recent demonstration of chemical-based cleaning of Cu
interconnects is expected to overcome disadvantages of physical Ar
sputtering process, such as chamfering and re-deposition on vias and
trenches. A number of studies on vapor-based reduction of copper oxide
under ambient pressure conditions and at temperatures below 350 °C using
hydrogen, ammonia, carbon monoxide, forming gas, acetic acid, formic
acid, and ethanol as reducing agents have been reported [5,6]. On the
other hand, Hydrazine (N2H4) can be used in the reduction of copper
oxide due to its higher reduction capability [7].
Inspired
by hydrazine’s unique characteristics, University of Texas at Dallas
and RASIRC have explored the feasibility of vapor-phase reduction of
copper oxide using anhydrous N2H4 to achieve an ideal metallic Cu film
in an ALD environment.
Due to the lack of volatile copper compounds, copper could not be patterned by the techniques of photoresist masking and plasma etching that had been used for aluminum. The inability to plasma etch copper meant that the whole metal patterning process had to be redesigned and the result was a process referred to as an additive patterning, also known as a "Damascene" or "dual-Damascene" process by analogy to a traditional technique of metal inlaying. [2]
However, the exposed Cu interconnects during via-opening and post CMP process are vulnerable to oxidation with water rinse and exposure to air, resulting in reliability degradation [3]. Therefore, additional process for reduction of copper oxide should be required. The cleaning of copper can be achieved by either physical Ar sputtering or chemical reduction process [4]. Recent demonstration of chemical-based cleaning of Cu interconnects is expected to overcome disadvantages of physical Ar sputtering process, such as chamfering and re-deposition on vias and trenches. A number of studies on vapor-based reduction of copper oxide under ambient pressure conditions and at temperatures below 350 °C using hydrogen, ammonia, carbon monoxide, forming gas, acetic acid, formic acid, and ethanol as reducing agents have been reported [5,6]. On the other hand, Hydrazine (N2H4) can be used in the reduction of copper oxide due to its higher reduction capability [7].
Inspired by hydrazine’s unique characteristics, University of Texas at Dallas and RASIRC have explored the feasibility of vapor-phase reduction of copper oxide using anhydrous N2H4 to achieve an ideal metallic Cu film in an ALD environment.
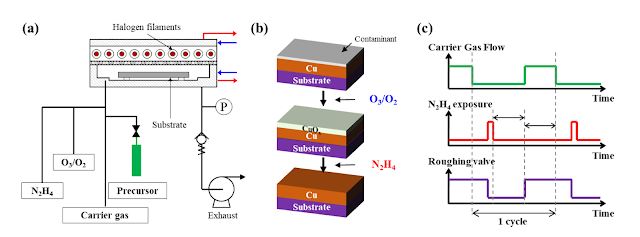
Figure 1. Schematic of (a) RTALD system, (b) Process sequence, and (c) representative time sequence of stop valve process.
In summary, it could be shown that following an ozone treatment (Figure 1) a N2H4 treatment could effectively reduce the Cu2O to metallic Cu(0) from 150 – 200 oC. In addition, there was no detection of intermediate materials (e.g. Cu3N, Cu(OH)2, CuH, etc.). The following possible thermodynamic reaction is given CuO + Cu2O + N2H4 à 3Cu + 2H2O(g) + N2(g)
The details of the study will be presented at AVS ALD2019 and future work will be on potential application to Ru and Co cleaning/reduction, which have become important interconnect metals for 14/16 nm Logic and below, especially at the highly scaled lower metallization levels (M0 to M4).
References
1. R. P. Chaukulkar, N. F. W. Thissen, V. R. Rai, and S. Agarwal, J. Vac. Sci. Technol. A, 32, 01A108 (2014).
2. Copper interconnects, Wikipedia LINK: https://en.wikipedia.org/wiki/Copper_interconnects
3. Y.-L. Cheng, C.-Y. Lee, and Y.-L. Huang, in Noble and Precious Metals-Properties, Nanoscale Effects and Applications, M. Seehar and A. Bristow, Editors, p. 216–250, Intechopen (2018).
4. C. K. Hu et al., Microelectron. Eng., 70, 406–411 (2003).
5. L. F. Pena, J. F. Veyan, M. A. Todd, A. Derecskei-Kovacs, and Y. J. Chabal, ACS Appl. Mater. Interfaces, 10, 38610–38620 (2018).
6. Y. Chang, J. Leu, B.-H. Lin, Y.-L. Wang, and Y.-L. Cheng, Adv. Mater. Sci. Eng., 2013, 1–7 (2013).
7. D. M. Littrell, D. H. Bowers, and B. J. Tatarchuk, J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases, 83, 3271–3282 (1987).



%20(1).png)
great
ReplyDeleteOn this time we know very well that we are suffering a great crisis for the pandemic. That's why we should always clean our house as well our offices. And for that you can go transfer maid agency singapore because this is the best place to hire transfer maid. Also from here you'll get all professional maids who can do every kind of work for you.
ReplyDeleteI'm living in Singapore for the last 15 years. For the last 3.5 years, I'm hiring a maid through the Universal Employment Agency. They are the best maid agency in Singapore & they can help you to choose the best helper for you easily.
ReplyDeleteWhen you need the best cleaning company at that time I would like to suggest you to visit cleaning company Ajax because from there you can easily get all professional cleaners and workers just at a simple and reasonale cost.
ReplyDeleteI think this is an informative post and it is very beneficial and knowledgeable. Therefore, I would like to thank you for the endeavors that you have made in writing this article. All the content is absolutely well-researched. Thanks.taxi luchthaven
ReplyDeleteI am grateful for this blog to distribute knowledge about this significant topic. Here I found different segments and now I am going to use these new instructions with new enthusiasm.Eco-friendly Promotional gifts
ReplyDelete